Principal Investigators: Richard Cairncross, Giuseppe Palmese, & Yossef Elabd (Drexel University, Chemical & Biological Engineering), Shri Ramaswamy (University of Minnesota, Biobased Products), Marc Hillmyer (University of Minnesota, Chemistry)
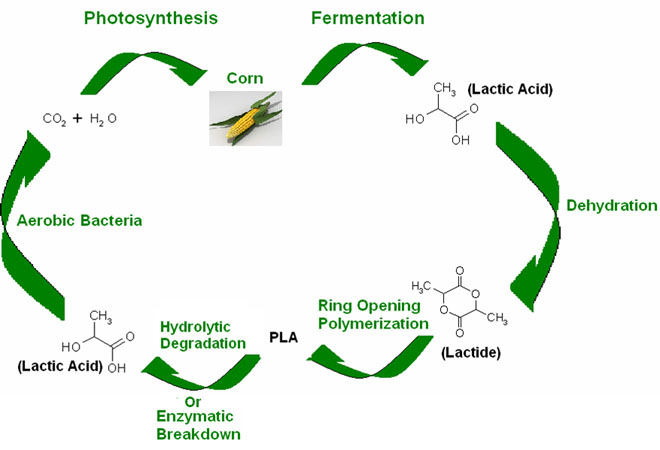
Bio-based polymers have numerous potential advantages over traditional polymers. Polylactide (PLA) is a biodegradable and environmentally friendly polyester that is made from renewable sources. However, the moisture barrier properties in PLA are still poorly understood. That is one of the major problems preventing PLA from being competitive with petroleum-based polymers in food packaging applications.
The objective of this research is to study water transport properties in PLA with the final goal to improve its moisture barrier properties. Specifically, water sorption and permeation in commercial and modified polylactide (PLA) with detailed mechanisms and characterization studies are being investigated. Modified PLA or PLA's derivatives were prepared by varying aliphatic content with different end groups, copolymerization, and polymer blending with composite particles. Water sorption in PLA was studied via the quartz crystal microbalance/heat conduction calorimetry (QCM/HCC) technique. Theoretical models were applied to compare with experimental results.
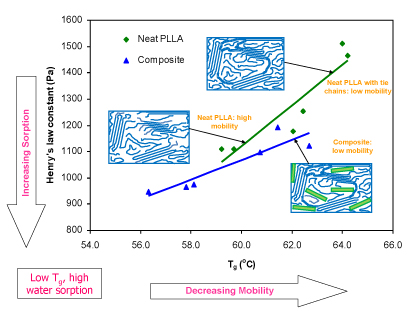
Water sorption and glass transition temperature of neat polylactide and polylactide-montmorillonite composites vary with heat treatment conditions. Although the crystallinity of all samples was similar, high heat treatment temperatures produce samples with lower glass transition temperatures (higher mobility) and higher sorption (lower Henry's Law constant). The trends for both neat polylactide and composite samples are the same, but composites samples exhibit higher mobility and higher sorption.
Graft copolymerization causes a negligible change in the amount of water sorption and exhibited similar evidence for the presence of water clusters with homo PLA. In addition, water sorption is affected by molecular weights, stereochemistry, end group compositions and fabricating nanocomposites. Heat treatment of PLA generates different morphologies and crystallinities and therefore plays an important role in determining the level of water sorption in PLA. Table 1 below shows a brief summary of how water sorption changes in several key samples. Each sorption isotherm is represented by a "solubility" which is the slope of the linear portion of the isotherm with (g water/g PLA) on the y axis and water activity on the x-axis. The % deviation indicates how much solubility of a sample deviates from solubility of the standard commercial amorphous PLA (PDLLA100k) which is an average value at three heat treatment conditions (0.007 g water/g PLA). Table 2 shows the permeability of some key samples, including the PLA-montmorillonite nanocomposites.
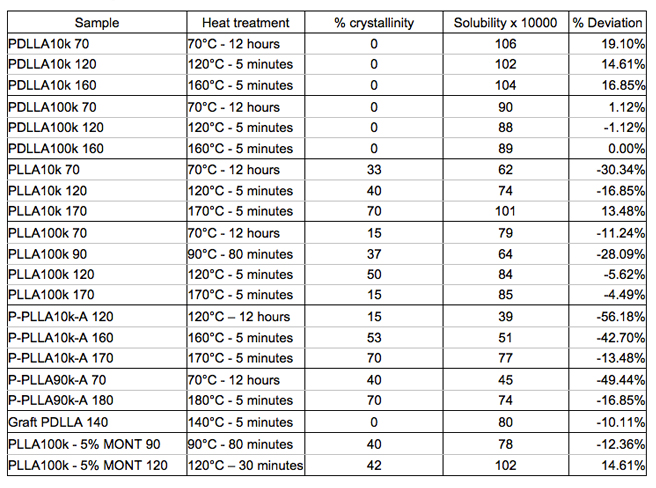
Table 1. Summary of sorption results in PLAs by varying molecular weight, stereochemistry and end group composition
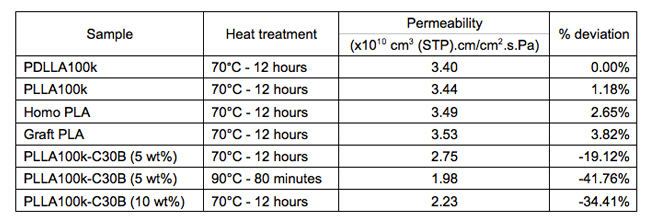
Table 2. Summary of permeation results in key samples, including nanocomposite samples at different heat treatments and concentrations of montmorillonite
Water sorption is a complex function of morphology, crystallinity, molecular weight and stereochemistry. Low molecular weight end-capped samples reduce the amount of water sorption significantly. A combination of end-group modification, low molecular weight and optimal heat treatment condition provides the lowest water sorption. Specifically, P-PLLA10k-A heat treated at 120°C absorbs ~50% less than commercial semi-crystalline PLLA at the same heat treatment condition. Fabricating PLLA-nanoclay composites increases the water sorption in PLLA due to the presence of the hydroxyl groups on the structure of nanoclays and the high mobility of the amorphous domain. However, it is shown that the water permeability in PLLA-nanoclay composites decreases significantly (34% reduction at 10 wt% of nanoclays). This is a strong evidence to show that the diffusion process is much slower in composite samples compared to neat PLLA samples. Furthermore, with the surface modification of nanoclays by oleic-acid coating, the dispersion of nanoclays in the polymer matrix is improved significantly, which leads to further improvement in water permeation as shown in solution-cast samples. Therefore, the good dispersion and alignment of nanoclays are the key factors in determining the moisture barrier properties of the composite membranes.
PLA Cycle
Applications of PLA

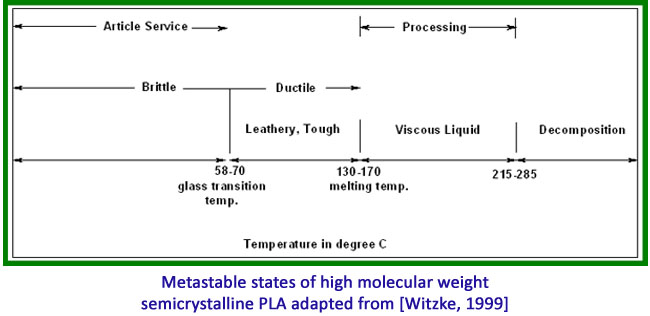
Thermal Properties of PLA